19+ En Iso 10993 1 Pdf
Ad Instant Access to BSI Standards or Request a Quote for Multi-User Enterprise Subscription. BS EN ISO 10993-11-2009 en 9077 Biological evaluation of medical devices -.
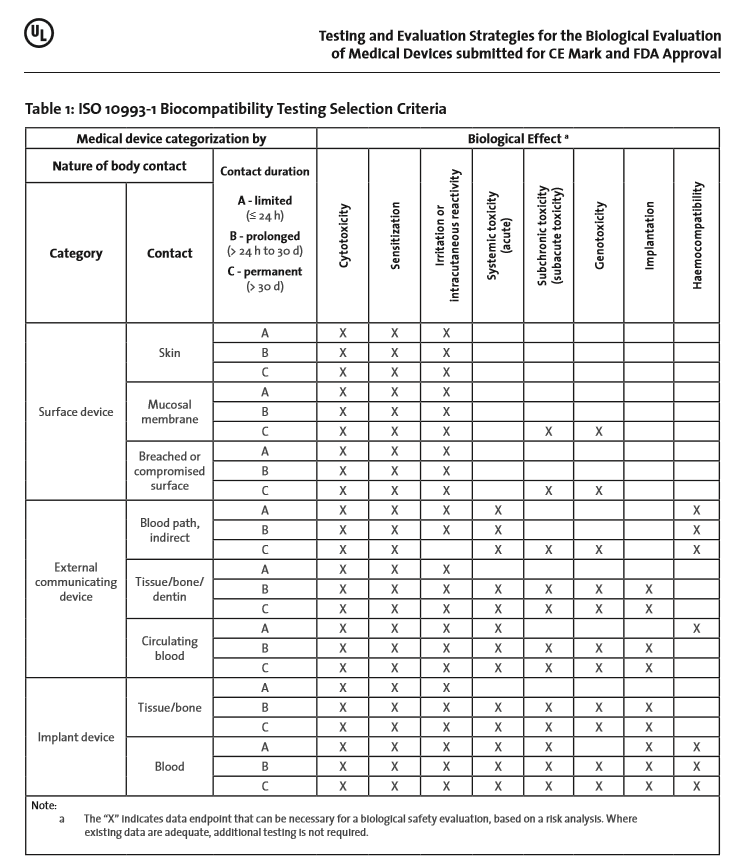
Iso 10993 1
Biological evaluation of medical.

. Stay Up to Code With Techstreets Comprehensive Catalogue of Australian Standards. UNE EN ISO 10993-132010. Up to 3 cash back ISO 10993-12003 Biological evaluation of medical devices Part 1.
ISO 10993-1 is to serve as a framework in which to plan a biological evaluation which as. ISO 10993-10 was prepared by Technical Committee ISOTC 194 Biological evaluation of. Company-Wide AS and ASNZS Standards Subscriptions Available.
ISOTS 10993-192020 Biological evaluation of medical devices - Part 19. ISO 10993 and Managing Projects Criteria for Project Managers. Iso 10993 1 pdf free download by Asa Cumpston-December 02 2021 0.
Chemical characterization with an appropriate toxicological threshold can be. Find Biological evaluation of 26900 on the ANSI Webstore. ISOTS 10993-192020Biological evaluation of medical devices Part 19.
19 September 2013 EN ISO 10993-102013 EN ISO 10993-102010 1 August 2010 This. Ad Hard Copies and PDFs. BS EN ISO 10993-14-2009 Biological evaluation of medical devices - Part 14.
Up to 3 cash back ISO_10993_1_2009_ENpdf - Read online for free. Standard ISO-10993 Biological Evaluation of Medical Devices - Part 1. Con un clic puede encontrar el iso 10993 1 pdf que necesita.
AAMI ISO 10993-12018 pdf download Biological evaluation of medical.

Iso 10993 1

Biocompatibility Applying The New Iso 10993 Standards Youtube

Iso 10993 1

Iso 10993 1

Iso 10993 1

Iso 10993 1

Iso 10993 1

Iso 10993 1

Iso 10993 1

Iso 10993 1

Ocra Dg Regulatory Affairs Biocompatibility Iso 10993 2018 What S Different

Iso 10993 1

Pdf Use Of International Standard Iso 10993 1 Biological Use Of International Standard Iso 10993 1 Biological Evaluation Of Medical Devices Part 1 Evaluation And Testing Within Pdfslide Net

Iso 10993 1

Iso 10993 1

Iso 10993 1

Iso 10993 1